Otrivine product range

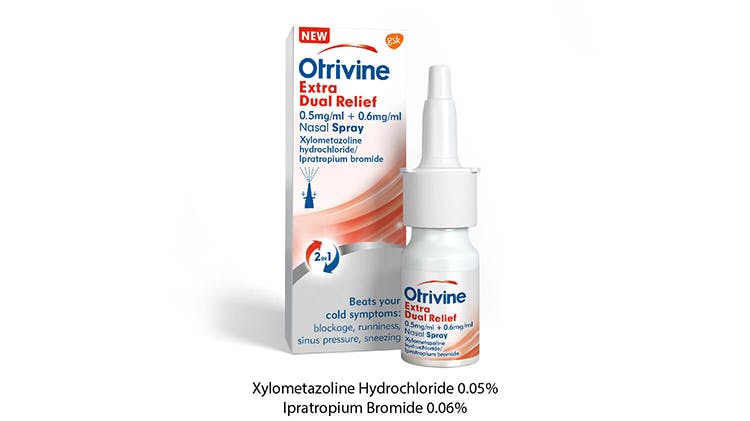
Otrivine Extra Dual Relief 0.5 mg/ml + 0.6 mg/ml Nasal Spray
A pharmacy-only medicine, available for you to supply and recommend in pharmacy, without a doctor’s prescription.
Suitable for use from 18 years and older
Do not use for more than 7 consecutive days.

Otrivine Natural – Daily Nasal Wash
Non medicated isotonic nasal wash with aloe vera.
3-in-1 daily nasal care – cleansing, moisturising, soothing. Cleans and restores the nose, allowing it to function as a natural filter.
100% natural – free of drugs, steroids and preservatives. Suitable for age 2 years+.

Otrivine Congestion Relief 0.1% Nasal Spray
Provides relief in as little as 2 minutes, lasts up to 10 hours
Relieves symptoms of nasal congestion in connection with the common cold, perennial and allergic rhinitis (including hayfever) sinusitis. Combines the instant freshness of menthol with the decongestant power of Otrivine to help relieve even heavy forms of congestion
Suitable for use from 12 years and older
Do not use for more than 7 consecutive days.

Otrivine Blocked Nose Relief Nasal Spray
Provides relief in as little as 2 minutes, lasts up to 10 hours
Relieves symptoms of nasal congestion in connection with the common cold, perennial and allergic rhinitis (including hayfever) sinusitis.
Suitable for use from 12 years and older
Do not use for more than 7 consecutive days.

Otrivine Adult Nasal Spray
Provides relief in as little as 2 minutes, lasts up to 10 hours
Relieves symptoms of nasal congestion in connection with the common cold, perennial and allergic rhinitis (including hayfever) sinusitis.
Suitable for use from 12 years and older
Do not use for more than 7 consecutive days.

Otrivine Adult Nasal Drops
Relieves symptoms of nasal congestion in connection with the common cold, perennial and allergic rhinitis (including hayfever) sinusitis.
Suitable for use from 12 years and older
Do not use for more than 7 consecutive days.

Otrivine Child Nasal Drops
Relieves symptoms of nasal congestion in connection with the common cold, perennial and allergic rhinitis (including hayfever) sinusitis. Lasts for up to 10 hours.
Suitable for use for 6 - 12 years
Do not use for more than 5 consecutive days.

Otricaps Blocked Nose and Sinusitis Relief 300mg/ 25mg/ 5mg Capsules, hard (paracetamol, caffeine, phenylephrine hydrochloride)
Symptomatic relief of nasal congestion and difficult breathing arising from this, sinusitis and its associated pain, acute nasal catarrh.
Also relieves other symptoms of cold and flu including, headache, fever, and sore throat.
Suitable for use from 16 years of age.
Should not be used for more than 7 consecutive days without advice from a healthcare professional.
Help your patients to manage their nasal congestion with Otrivine
Otrivine Extra Dual Relief
Find out how Otrivine Extra Dual Relief can help your customers and patients.



