Otrivine Extra Dual Relief 0.5mg/ml + 0.6mg/ml, Nasal Spray

Recommend Otrivine Extra Dual Relief to help relieve nasal congestion symptoms fast
Unique formulation for a blocked and runny nose from colds
- Pharmacy-only medicine*
- Nasal congestion, runny nose, sinus pressure and sneezing are treated simultaneously
- Only product to contain a combination of xylometazoline hydrochloride and ipratropium bromide
Otrivine Extra Dual Relief contains two active ingredients which work to relieve different nasal symptoms
Xylometazoline hydrochloride (0.5 mg/ml)
- Reduces the diameter of blood vessels in the nose to reduce nasal mucosal swelling
- Has a decongestant effect
- Fast effect usually obtained after 5-10 minutes and lasts for 6-8 hours
Ipratropium bromide (0.6 mg/ml)
- Reduces nasal mucus secretion
- Fast effect usually obtained within 15 minutes and lasts for an average of 6 hours
For news, updates and access to more resources please register now to be on our mailing list.
*available without prescription
Download additional resources for your pharmacy

Otrivine Extra Dual Relief ‘How to recommend in Pharmacy’ booklet
This pharmacy guide provides information on differences between topical medication for nasal symptoms of the common cold and for ongoing nasal problems, active ingredients, mode of action, usage and recommendation advice for Otrivine Extra Dual Relief.

Otrivine Extra Dual Relief Patient Leaflet
This customer leaflet provides information on the symptoms of a cold and provides advice including the use of Otrivine sprays with instruction for use.

Otrivine Range Piece
This pharmacy guide provides condensed information on who Otrivine is for, active ingredients, mode of action, usage and recommendation advice for Otrivine Extra Dual Relief.
About Otrivine Extra Dual Relief
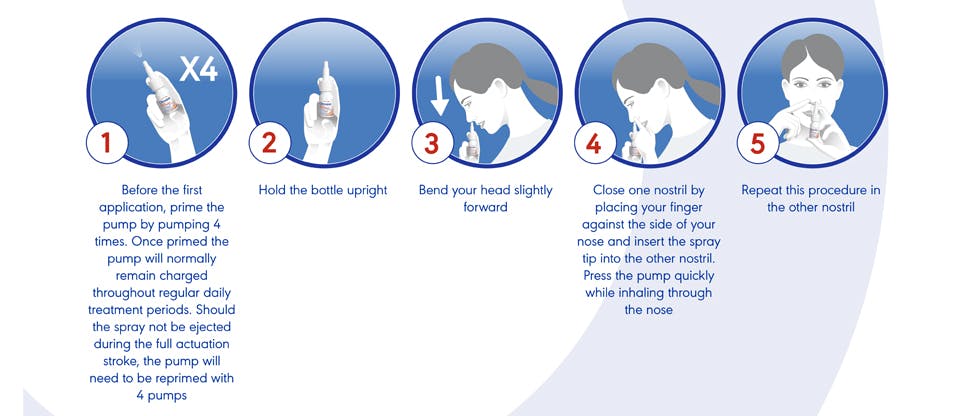
Instructions for use
- One puff in each nostril up to 3 times per day
- Leave at least 6 hours between doses
- Not more than 3 applications daily into each nostril
- The product should not be used for more than 7 days
- Always blow your nose before using the nasal spray
Suitable for use from 18 years and older.
Do not use for more than 7 consecutive days.
Product Information
Otrivine Extra Dual Relief 0.5 mg/ml + 0.6 mg/ml nasal spray, solution (xylometazoline hydrochloride, ipratropium bromide). Indications: Symptomatic treatment of nasal congestion and rhinorrhea in connection with common colds. Dosage: Adults: 1 puff in each nostril, up to 3 times daily, with at least 6 hours between doses. Maximum 3 applications daily into each nostril. Do not exceed the stated dose. Do not use for longer than 7 days. Geriatrics: Limited experience of use in patients above 70 years of age. Contraindications: Children and adolescents under 18 years. Known hypersensitivity to xylometazoline hydrochloride, ipratropium bromide, any of the excipients, atropine or atropine-like substances. Patients with glaucoma, rhinitis sicca, atrophic rhinitis or following trans-sphenoidal hypophysectomy or surgery exposing the dura mater. Warnings and precautions: Use with caution in people with: hypertension, cardiovascular disease, long QT syndrome, hyperthyroidism, diabetes mellitus, prostatic hypertrophy, bladder stenosis, pheochromocytoma, on monoamine oxidase inhibitors treatment or tri/tetra cyclic antidepressants (or have been on either within the last two weeks), on beta 2-agonist treatment, cystic fibrosis. Use with caution in patients predisposed to angle closure glaucoma, epistaxis or paralytic ileus. Immediate hypersensitivity including urticaria, angioedema and rash may occur. Caution in people sensitive to adrenergic substances. Avoid contact with eyes. Side effects: See SPC for full details. Very common: epistaxis. Common: dysgeusia, rhinalgia, nasal congestion, headache, nasal dryness, nasal discomfort, nausea, application site burn, dizziness, throat irritation, dry throat, dry mouth. Uncommon: insomnia, parosmia, tremor, eye irritation, dry eye, palpitations, tachycardia, nasal ulcer, sneezing, dyspepsia, fatigue, corneal oedema, cough. Price: £6.66 (ex. VAT) Legal Status: P. Licence Number: PL 15545/0002. Licence Holder: GlaxoSmithKline Dungarvan Limited, Knockbrack, Dungarvan, County Waterford, Ireland. Date of revision: October 2023
Recommend Otrivine Extra Dual Relief - Unique formulation for a blocked and runny nose from colds

The Otrivine range
Find out how the Otrivine range can help your customers and patients.
Recommend Otrivine Extra Dual Relief - Unique formulation for a blocked and runny nose from colds

E-Learning Modules
Sign in or register to access our e-learning modules on allergic rhinitis. This interactive module allows you to track your progress and can be accessed 24/7.

