Voltaren Arthritis Pain Safety Profile

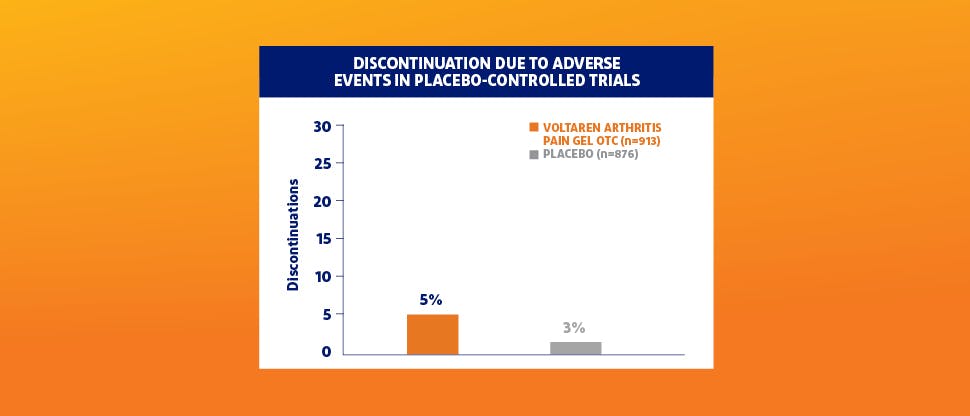
Adverse events were comparable to placebo in placebo-controlled trials1,2
The only adverse reactions that occurred in >1% of patients with greater frequency with Voltaren® Arthritis Pain gel OTC vs placebo were application-site reactions (Voltaren® Gel 7%, placebo 2%).1,2
See Voltaren® Arthritis Pain gel OTC Drug Facts for NSAID class warnings.
Learn more about Voltaren® Arthritis Pain—the #1 doctor recommended topical pain brand*5

How does Voltaren® Arthritis Pain gel OTC stack up against the competition?
Voltaren® Arthritis Pain compared with oral and topical analgesics.
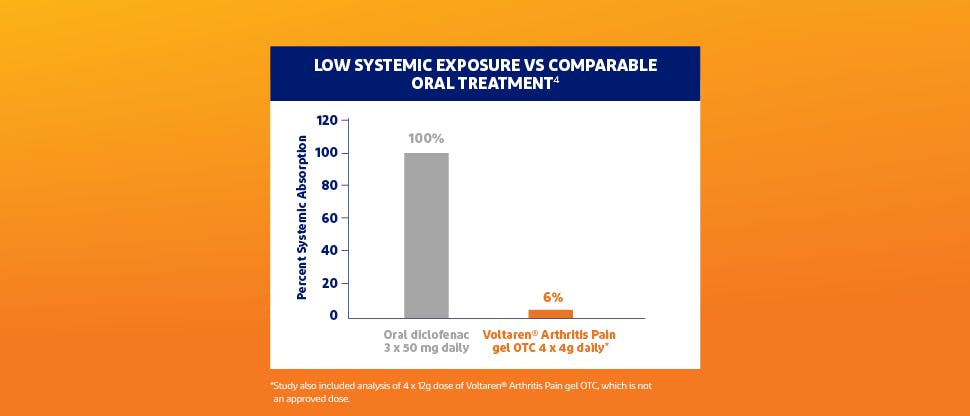
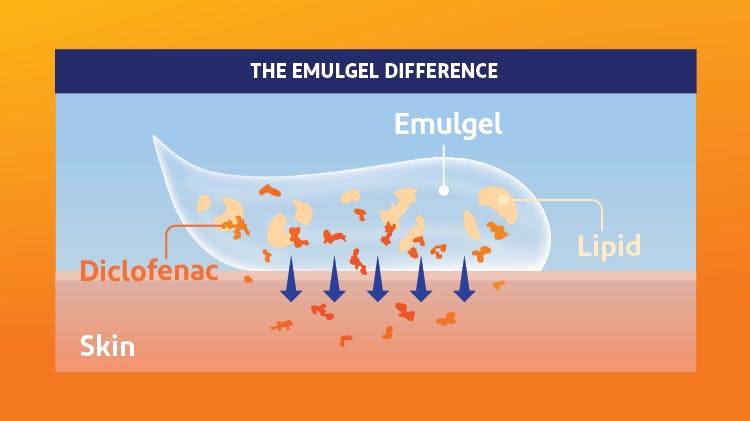
How does Voltaren® Arthritis Pain gel OTC penetrate the skin?
The science behind Voltaren® Arthritis Pain and its MOA.
Voltaren® Arthritis Pain gel OTC Drug Facts
The drug facts contain all you need to know about dosing and administration.
How to treat OA Pain
The latest OA treatment guidelines from the American College of Rheumatology.