Nexium Control overview

Ireland’s No 1 pharmacy only 24 hour heartburn protection treatment1
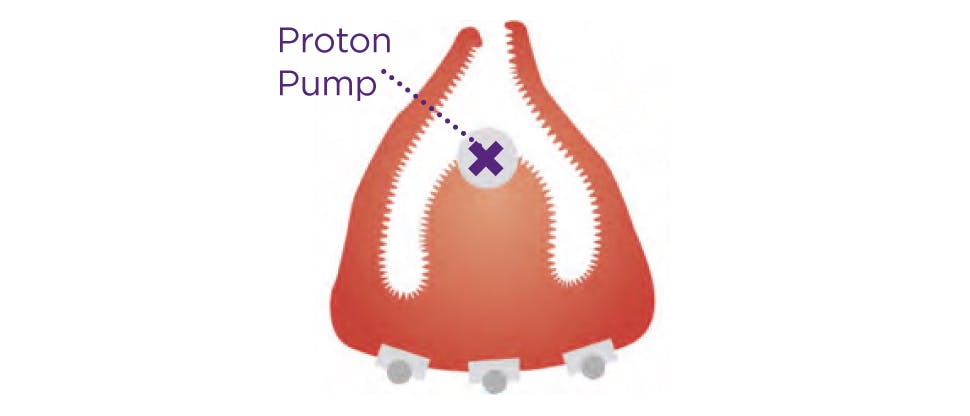
Unlike antacids and alginates that treat the symptoms of heartburn, Nexium Control tackles the cause by targeting acid directly at the source providing long-lasting protection.
For news, updates and access to more resources and webinars please register now to be on our mailing list.
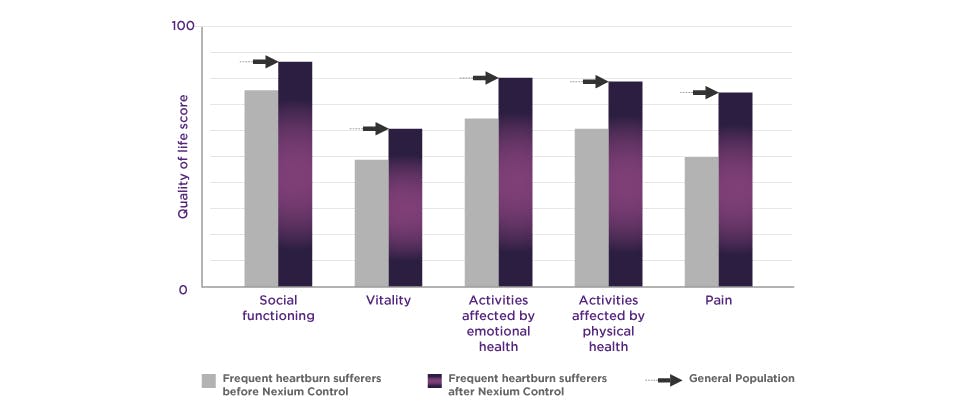
Nexium Control – Clinically proven protection for frequent heartburn

Long lasting protection
With just one pill a day Nexium Control provides up to 24 hour protection for frequent heartburn sufferers.

Clinically proven
8 out of 10 people reported first resolution within the first week of treatment with Nexium Control.

Nighttime relief
4 out of 5 nights were heartburn free for patients using Nexium Control.
Nexium Control – Your first line recommendation for frequent heartburn
Find out more about frequent heartburn
Explore videos, product guides and other resources to support you and your team when recommending Nexium Control for frequent heartburn. Click here
Recommend Nexium Control for your patients with frequent heartburn

Nexium Control 28 tablet pack
For patients with recurrent frequent heartburn who have used esomeprazole successfully and seek the value and convenience of a larger pack.
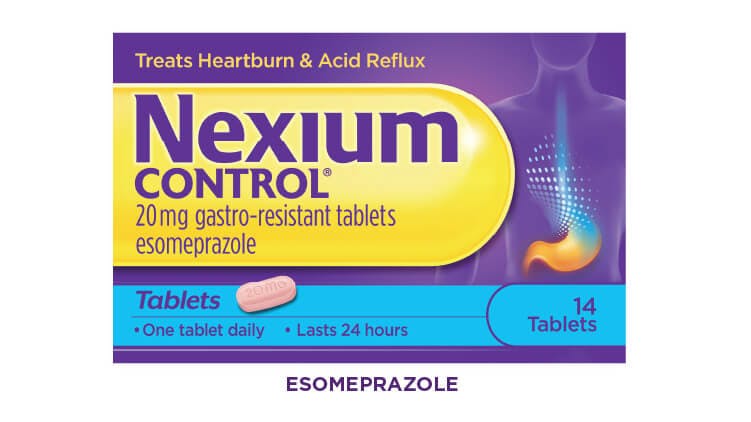
Nexium Control 20mg Gastro-Resistant Tablets (Esomeprazole)
Nexium Control 7 tablet pack is an ideal trial pack with one-week supply. Nexium Control 14 tablet pack offers the same protection and offers a 2-week treatment course.
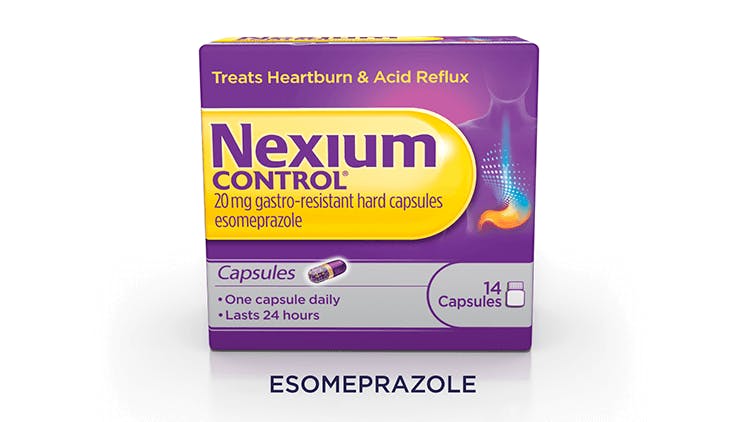
Nexium Control 20mg Gastro-Resistant Capsules (Esomeprazole)
Easy-to swallow mini capsules – 53% smaller than tablet* in a portable bottle. Complete 14-day treatment course.
*based on volume
Ireland’s No 1 pharmacy only 24 hour heartburn protection treatment1
Heartburn
Find out more about frequent heartburn including a profile of a patient who may suffer from the condition.
Sign up for updates
For news, updates and more, click the link below to sign up and be on our mailing list
Educational resources
Access educational resources to support your team’s understanding of heartburn and its management.